
The more concentrated ammonia pushes the equilibrium even further to the right, lowering the silver ion concentration even more. This occurs with silver chloride, and with silver bromide if the ammonia is concentrated. If the adjusted silver ion concentration multiplied by the halide ion concentration is less than the solubility product, some precipitate dissolves to restore equilibrium. The effect of adding the ammonia is to lower this concentration still further. The equation for this reaction is given below:Ī solution in contact with one of the silver halide precipitates contains a very small concentration of dissolved silver ions. This is a reversible reaction, but the complex is very stable, and the position of equilibrium lies well to the right. The ammonia combines with silver ions to produce a complex ion called the diamminesilver(I) ion, +.

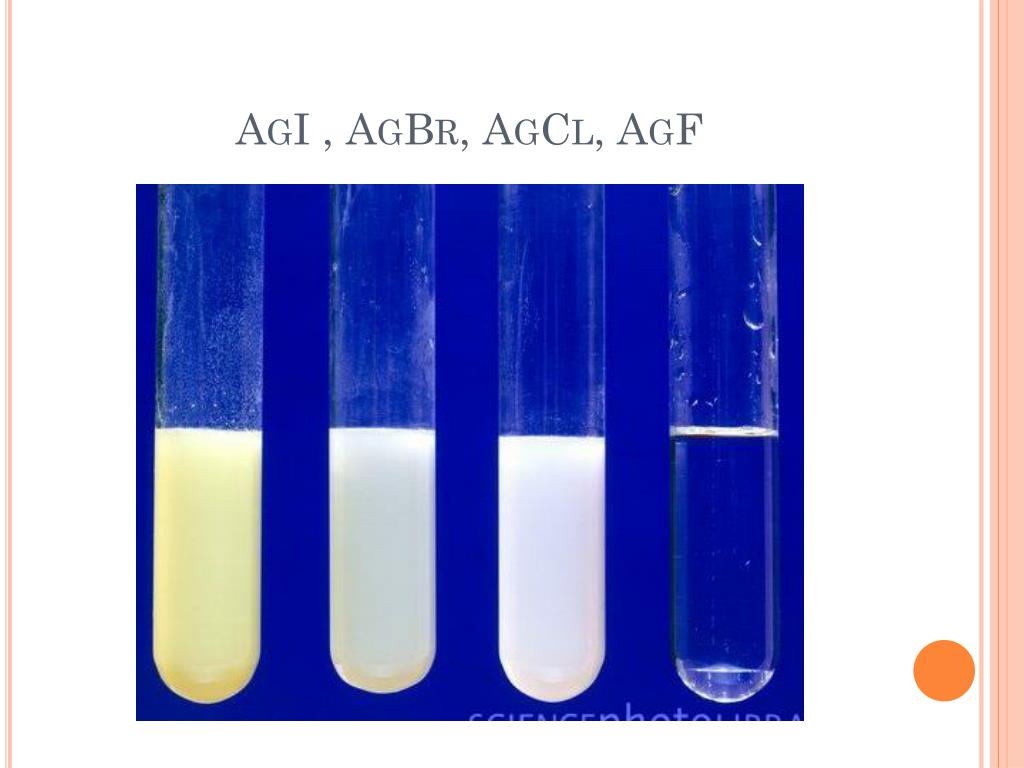
The compounds are all quite insoluble, but become even less so down the group. The table below lists solubility products from silver chloride to silver iodide (a solubility product for silver fluoride cannot be reported because it is too soluble). 100 color(red)(cancel(color(black)('L solution'))) (7.07 10(-4)color(white)(.)'moles AgBr')/(1color(red)(cancel(color(black)('L solution')))) color(darkgreen)(ul(color(black)(7 10(-2)color(white)(.)'moles AgBr'))) The answer is rounded to one significant figure, the number of sig figs you have for the volume of water. You can sort out which precipitate you have by adding ammonia solution. Enough solid is always precipitated to lower the ionic product to the solubility product. Various precipitates may be formed from the reaction between the silver and halide ions: Confirming the precipitates It is actually quite difficult to distinguish between these colors, especially if there isn't much precipitate. If the product of the concentrations exceeds this value, a precipitate is formed.Įssentially, the product of the ionic concentrations is never greater than the solubility product value.If the product of the concentrations of ions is less than the solubility product, no precipitate is formed.The square brackets indicate molar concentrations, with units of mol L -1. For the silver halides, the solubility product is given by the expression: This value is known as the solubility product. A precipitate forms if the concentrations of the ions in solution in water exceed a certain value, unique to every compound. There are no absolutely insoluble ionic compounds. Precipitate is insoluble in ammonia solution of any concentration Precipitate is almost unchanged using dilute ammonia solution, but dissolves in concentrated ammonia solution to give a colorless solution Precipitate dissolves to give a colorless solution Precipitates will form and disappear.\)Ĭonfirming the precipitate using ammonia solutionĪmmonia solution is added to the precipitates. Precipitate of calcium hydroxide will be of A) Green color B) Blue color C) Brown color D) White color. On moving down in the group the atomic size of the increases, the order is. Q8- Color of Solid magnesium is A) Dark grey B) Silver grey C) Black D) Whitish silver Q9- Consider equations: Ca²(aq) + 2OH(aq)Ca(OH) (s). Starting with 1 mL of sodium carbonate add the solutions consecutively to the water in the order listed in the materials section. The color of the compound AgCl is white but silver salt of AgBr and AgI are colored.Add 200 mL water and a stirring bar to the 600 mL beaker and begin stirring.
#AGBR PRECIPITATE COLOR SKIN#


 0 kommentar(er)
0 kommentar(er)
